- A
- B
- C
- D
- E
- F
- G
- H
- I
- J
- K
- L
- M
- N
- O
- P
- Q
- R
- S
- T
- U
- V
- W
- X
- Y
- Z
API名稱 : Tazobactam
- Tacrolimus
- Tadalafil
- Tafamidis
- Tafenoquine
- Tafluprost
- Talampicillin
- Talazoparib
- Tamoxifen
- Tamsulosin
- Tandospirone
- Tanespimycin
- Tapentadol
- Tartrazine
- Tasimelteon
- Taurine
- Taurodeoxycholic Acid
- Taurolidine
- Taurolithocholic Acid
- Tavaborole
- Taxifolin
- Tazarotene
- Tazemetostat
- Tazobactam
- Tebapivat
- Tebipenem Pivoxil
- Tebuconazole
- Tecovirimat
- Tedizolid
- Teduglutide
- Teferdin
- Tegafur
- Tegaserod
- Tegoprazan
- Teicoplanin
- Telaprevir
- Telbivudine
- Telmisartan
- Telotristate Ethyl
- Temazepam
- Temocapril
- Temozolomide
- Temsirolimus
- Teneligliptin
- Tenofovir
- Tenoxicam
- Tepotinib
- Teprenone
- Terazosin
- Terbinafine
- Terbutaline
- Terconazole
- Terephthalic Acid
- Teriflunomide
- Teriparatide
- Terlipressin
- Ternidazole
- Terpin
- Tesofensine
- Testosterone
- Testosterone Cypionate
- Tetrabenazine
- Tetracaine
- Tetracosactide
- Tetracycline
- Tetraxetan
- Tetrazepam
- Tetryzoline Hydrochloride
- Tezacaftor
- Thalidomide
- Theophylline
- Thiabendazole
- Thiamazole
- Thiamine
- Thiamphenicol
- Thimerosal
- Thiocolchicoside
- Thioctic Acid
- Thiopental
- Thioridazine
- Thiorphan
- Thiotepa
- Thiothixene
- Thymol
- Thymosin
- Tiagabine
- Tiamulin
- Tianeptine
- Tibolone
- Ticagrelor
- Ticlodipine
- Ticlopidine
- Tigecycline
- Timolol
- Tinidazole
- Tioconazole
- Tiopronin
- Tiotropium
- Tirofiban
- Tiropramide
- Tirzepatide
- Tivozanib
- Tizanidine
- Tizoxanide
- Tobramycin
- Tocopheryl Acetate
- Tofacitinib
- Tofisopam
- Tolcapone
- Tolfenamic Acid
- Tolnaftate
- Tolperisone
- Tolterodine
- Tolvaptan
- Topiramate
- Topotecan
- Torasemide
- Toxaphene
- Trabectedin
- Tramadol
- Tramadol
- Trametinib
- Trandolapril
- Tranexamic Acid
- Tranylcypromine
- Travoprost
- Trazodone
- Trehalose
- Trelagliptin
- Treosulfan
- Treprostinil
- Tretinoin
- Triamcinolone
- Triamcinolone Acetonide
- Triamterene
- Tribenoside
- Trichlormethiazide
- Trichodesmine
- Triclabendazole
- Trientine
- Trifluoperazine
- Trihexyphenidyl
- Trilaciclib
- Trilostane
- Trimebutine
- Trimetazidine
- Trimethobenzamide
- Trimethoprim
- Trimipramine
- Triprolidine
- Triptorelin
- Triricinolein
- Trofinetide
- Tropicamide
- Trospium Chloride
- Tryptophan
- Tryptophol
- Tuaminoheptane Sulfate
- Tucatinib
- Tulathromycin
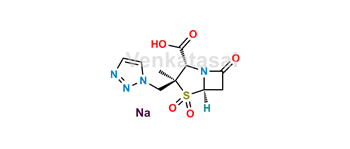
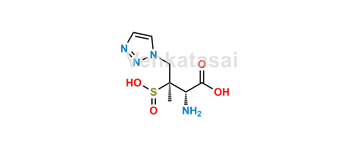
Tazobactam
Venkatasai is a leading provider of pharmaceutical reference standards, specializing in the supply of high-purity Tazobactam reference materials. Our portfolio includes both pharmacopeial and non-pharmacopeial impurities, metabolites, stable isotope-labeled compounds, and nitrosamine derivatives (N-NO products). These reference standards play a crucial role in pharmaceutical R&D, aiding in formulation development, ANDA/DMF submissions, quality control, method validation, stability studies, and impurity profiling—including the identification and assessment of potentially genotoxic impurities.
Each Tazobactam-related product is rigorously characterized and delivered with a detailed Certificate of Analysis (COA) and comprehensive analytical data to ensure regulatory compliance. Upon request, we also offer EP/USP traceable standards. To maintain the highest quality standards, all materials are subject to periodic re-testing and continuous quality monitoring.