Venkatasai is a leading innovator in the field of pharmaceutical reference standards, offering high-quality Reference Standards of Abacavir, including both pharmacopeial and non-pharmacopeial impurities, metabolites, stable isotope-labeled compounds, and nitrosamines (N-NO products). Our Abacavir impurity reference standards play a vital role in pharmaceutical research, supporting product development, ANDA and DMF filings, quality control (QC), method validation, and stability studies. They are also instrumental in identifying unknown impurities and evaluating genotoxic potential. All Abacavir-related products are thoroughly characterized and supplied with comprehensive Certificates of Analysis (COA) and analytical data that comply with regulatory standards. EP/USP traceable standards can also be provided upon request. Furthermore, all supplied products undergo periodic re-testing to ensure continued quality and reliability.
X
Venkat Sai Life Science
x
- Upcoming Expo
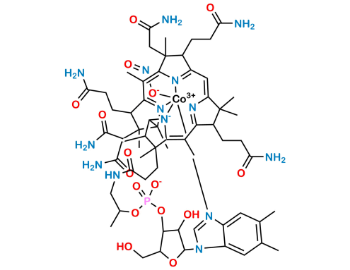
Hydroxocobalamin Acetate Related Products
CAT No: VS-H016000
CAS No: 13422-51-0
Mol.F.: C62H89CoN13O15P
Mol.Wt.: 1346.4
Status: Custom Synthesis
CAT No: VS-H016001
CAS No: 22465-48-1
Mol.F.: C64H91CoN13O16P
Mol.Wt.: 1388.4
Status: Custom Synthesis
CAT No: VS-H016002
CAS No: 20623-13-6
Mol.F.: C62H88CoN14O16P
Mol.Wt.: 1375.4
Status: Custom Synthesis