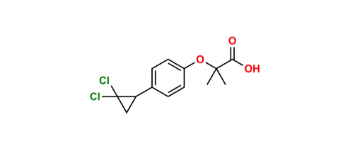
Ciprofibrate
Venkatasai is a leading innovator in the field of pharmaceutical reference standards, offering high-quality Reference Standards of Abacavir, including both pharmacopeial and non-pharmacopeial impurities, metabolites, stable isotope-labeled compounds, and nitrosamines (N-NO products). Our Abacavir impurity reference standards play a vital role in pharmaceutical research, supporting product development, ANDA and DMF filings, quality control (QC), method validation, and stability studies. They are also instrumental in identifying unknown impurities and evaluating genotoxic potential. All Abacavir-related products are thoroughly characterized and supplied with comprehensive Certificates of Analysis (COA) and analytical data that comply with regulatory standards. EP/USP traceable standards can also be provided upon request. Furthermore, all supplied products undergo periodic re-testing to ensure continued quality and reliability.
Ciprofibrate
Ciprofibrate EP Impurity A
Ciprofibrate EP Impurity B
Ciprofibrate EP Impurity C
Ciprofibrate EP Impurity D
Ciprofibrate EP Impurity E
Categories
- Cabazitaxel
- Cabergoline
- Cabotegravir
- Cabozantinib
- Caffeine
- Calanolide
- Calcifediol
- Calcipotriol
- Calcitriol
- Calcobutrol
- Camptothecin
- Canagliflozin
- Candesartan
- Cangrelor
- Capecitabine
- Capsaicin
- Captopril
- Carbamazepine
- Carbidopa
- Carbinoxamine
- Carbocisteine
- Carboplatin
- Carboprost Tromethamine
- Carbovir
- Carebastine
- Carfilzomib
- Carglumic Acid
- Cariprazine
- Carisoprodol
- Carmustine
- Carprofen
- Carvedilol
- Caspofungin
- Cefacetrile
- Cefaclor
- Cefadroxil
- Cefalexin
- Cefalonium
- Cefalotin
- Cefazedone
- Cefazolin
- Cefdinir
- Cefditoren Pivoxil
- Cefepime
- Cefixime
- Cefoperazone
- Cefotaxime Sodium
- Cefotiam
- Cefoxitin
- Cefozopran
- Cefpodoxime Proxetil
- Cefprozil Monohydrate
- Ceftaroline Fosamil
- Ceftazidime Pentahydrate
- Ceftiofur
- Ceftizoxime Sodium
- Ceftriaxone
- Cefuroxime
- Celecoxib
- Celiprolol Hydrochloride
- Ceramide
- Ceritinib
- Cetirizine
- Cetrorelix
- Cetylpyridinium Chloride
- Cevimeline
- Chenodeoxycholic Acid
- Chicoric Acid
- Chlorambucil
- Chloramphenicol
- Chlorhexidine
- Chlorhexidine Gluconate Solution
- Chlormadinone
- Chloroquine
- Chlorphenamine
- Chlorpromazine
- Chlorprothixene
- Chlortalidone
- Cholecalciferol
- Cholesterol
- Cibenzoline
- Ciclesonide
- Ciclopirox
- Cidofovir
- Cilastatin
- Cilazapril
- Cilnidipine
- Cilostazol
- Cimetidine
- Cinacalcet
- Cinchocaine
- Cinchonidine
- Cinnarizine
- Ciprofibrate
- Ciprofloxacin
- Cisapride
- Cisatracurium
- Cisplatin
- Citalopram
- Citicoline
- Cladribine
- Clarithromycin
- Clavulanate
- Clemastine
- Clevidipine
- Clidinium Bromide
- Clindamycin
- Clioquinol
- Clobenzorex
- Clobetasol Propionate
- Clobetasone Butyrate
- Clobutinol Hydrochloride
- Clodronate
- Clofarabine
- Clofazimine
- Clomiphene
- Clomipramine
- Clonidine
- Clonixin
- Clopidogrel
- Cloprostenol
- Clorazepate
- Clotrimazole
- Cloxacillin
- Clozapine
- Cobicistat
- Cobimetinib
- Colchicine
- Colesevelam
- Copanlisib
- Cortexolone
- Corticosterone
- Corydaline
- Creatinine
- Crisaborole
- Crizotinib
- Cromolyn
- Crotamiton
- Cyamemazine
- Cyanocobalamin
- Cyclizine
- Cyclobenzaprine
- Cyclophosphamide
- Cycloserine
- Cyclosporin
- Cyproheptadine
- Cyproterone
- Cytarabine
- Cytidine
- Cytosine
- Cetilistat
- Chlordiazepoxide
- Chlormezanone
- Cinitapride
- Clobazam
- Clonazepam
- Chlorphenesin Carbamate
- Caffeic Acid
- Cyclopentolate
- Calcium Saccharate
- Calcium Gluconate Monohydrate
- Campesterol
- Cannabidiol
- Carbazochrome
- Carbetocin
- Capmatinib
- Carbimazole
- Caryophyllene Oxide
- Cascaroside
- Cedazuridine
- Cefpirome Sulfate
- Cefradine
- Cefteram
- Ceftobiprole
- Ceftolozane
- Cenobamate
- Cephapirin
- Cerivastatin
- Cetrimide
- Chlorpropamide
- Chlorpyrifos
- Chlorquinaldol
- Chlorthion
- Chlorzoxazone
- Clascoterone
- Cloperastine
- Cloxazolam
- Codeine
- Colestipol
- Colfosceril Palmitate
- Codeinone
- Cornigerine
- Cortisone
- Cosyntropin
- Coumarin
- Creatine
- Curcumin
- Cyantraniliprole
- Cyclamate
- Cyprodinil
- Cyphenothrin
- CataCXium A
- Cytisine
- Chlorobutanol
- Camphor
- Chondroitin Sulfate
- Camylofin
- Candicidin
- Cannabigerol
- Carvone
- Catharanthine
- Cathine
- Cefetamet
- Ceftibuten
- Chlorocresol
- Chloroxylenol
- Choline Chloride
- Clemizole
- Clenbuterol Hydrochloride
- Clorsulon
- Capivasertib
- Clothianidin
- Coumatetralyl
- Cyhalothrin
- Cyproconazole
- Cysteamine
- Camizestrant
- Chlorcyclizine Hydrochloride
- Cafedrine
- Campestanol
- Carbodenafil
- Caricaxanthin
- Carteolol
- Chlorothiazide
- Chlorantraniliprole
- Cefquinome
- Cimaterol
- Cimbuterol
- Clenpenterol
- Clethodim
- Clofedanol
- Closantel
- Cefotetan
- Colistimethate
- Cefodizime
- Cefmetazole
- Cephalosporin C
- Clofentezine
- Calcitonin Salmon
- Cefovecin
- Chloropyramine
- Colistin